Feb 15, 2026

Lightweight design is a core advantage of cylinders compared to other drive systems. This characteristic is becoming increasingly important in cutting-edge automation fields, such as industrial robots and manipulators requiring precise end-effector control. These applications cannot bear excessive weight themselves, making pneumatic cylinders the optimal choice.
The core of cylinder lightweighting lies in the fact that many of its key components can be made from aluminum instead of iron, such as the barrel, end cover, and piston. However, this substitution also brings some challenges:
1. Insufficient Hardness: The relatively poor hardness of aluminum alloys makes them prone to scratching and wear. During assembly, maintenance, or operation, if dust enters the cylinder, the inner wall is easily scratched, leading to air leakage and reduced efficiency.
Furthermore, under long-term reciprocating motion, friction with the piston seals causes gradual wear of the inner wall dimensions, eventually exceeding tolerance limits. This results in seal failure, cross-port leakage, and loss of power.
2.Poor Corrosion Resistance: This is the most critical and apparent drawback. The naturally formed oxide film on aluminum alloy surfaces is extremely thin (only a few nanometers). In humid, saline, or acidic/alkaline environments, corrosive agents like chloride ions easily penetrate this natural film, causing pitting corrosion on the surface. These corrosion pits become stress concentration points and crack initiation sites.
To address these issues, anodizing treatment is applied to aluminum alloy cylinder barrels.
Anodizing is a process of forming a dense oxide film on a metal surface through chemical or electrochemical methods. Among these, anodic oxidation (anodizing) via electrochemical means is the most widely used and important surface treatment technology for aluminum alloys.
The anodizing process differs from aluminum's natural oxidation. Natural oxidation is a spontaneous reaction of aluminum in air, forming a dense oxide film only a few nanometers thick, which prevents further oxygen ingress. However, this film is too thin and soft to withstand mechanical wear and harsh corrosive environments.
In contrast, the anodizing process removes the natural oxide film and generates an oxide layer tens of micrometers thick on the aluminum surface through a method similar to "electrolysis." This overcomes the inherent drawbacks of pure aluminum or aluminum alloys being "soft, prone to wear, and susceptible to corrosion."
The workpiece to be treated is mounted on a fixture made of titanium or aluminum alloy. After a series of pre-treatment steps including water rinsing, alkaline etching, neutralization, and chemical polishing, it is finally immersed in an electrolytic bath containing sulfuric or oxalic acid.
In anodizing, aluminum serves as the anode, and lead (or inert materials like graphite, stainless steel) serves as the cathode. When current is applied, aluminum atoms at the anode lose electrons to become aluminum ions. Simultaneously, water molecules dissociate under the electric field, generating hydrogen ions and oxygen ions.
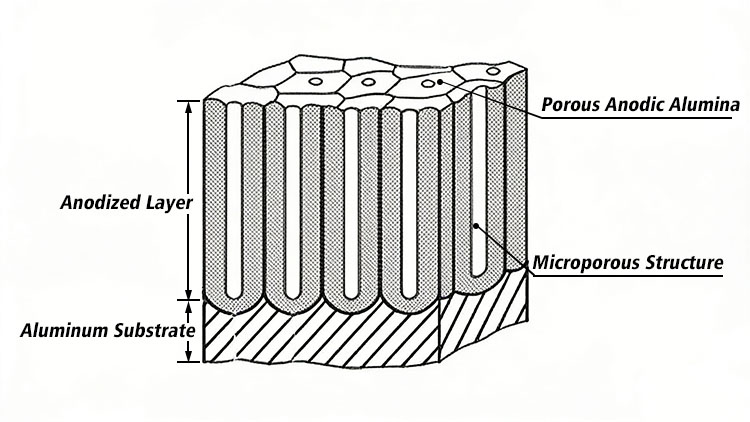
Aluminum ions and oxygen ions combine to form a honeycomb-like porous structure of aluminum oxide. This oxide layer thickens both inward into the metal and outward, eventually forming a thick alumina layer integrally bonded with the aluminum substrate.
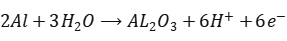
The alumina layer does not thicken indefinitely. While the electric field in the solution promotes the oxidation reaction, it also promotes the chemical dissolution of alumina by the electrolyte. After a period, a dynamic equilibrium is reached between the growth rate and dissolution rate. The alumina layer also becomes smoother and more uniform through repeated growth and dissolution.
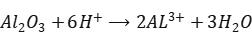
At the cathode, the hydrogen evolution reaction occurs:
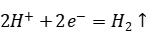
After anodizing, the workpiece requires rinsing with pure water. It can then undergo dyeing and sealing treatments to further enhance appearance and performance. Finally, it is dried to prevent watermarks on the surface.
◆ Significantly Improved Corrosion Resistance: The thick, dense oxide film acts as a perfect chemically inert barrier, resisting erosion from various media including atmosphere, seawater, acids, and alkalis.Greatly Enhanced Wear Resistance: Especially for hard anodized layers, the high hardness drastically reduces frictional wear and extends component life.
◆ Excellent Decorative Properties: The porous film can adsorb various organic dyes or inorganic pigments, achieving rich, durable, and aesthetically pleasing colors. For example, cylinder end caps often use black anodizing as a substitute for paint that is prone to chipping.
◆ Good Electrical Insulation: Alumina is an excellent electrical insulating material. The anodized layer can serve as an insulating coating.
◆ Enhanced Coating Adhesion: The chemically oxidized film features a porous microstructure. As a primer, it allows paints and powder coatings to adhere firmly.
It can be said that the anodizing process is indispensable in cylinder barrel production. It allows aluminum cylinders to retain their core advantages of being lightweight, having good thermal conductivity, and being easy to machine, while also gaining additional ceramic-like properties such as wear resistance, corrosion resistance, aesthetic appeal, and insulation.
(FK9009)
 How to Install a 5/2 Solenoid Valve onto a Manifold
How to Install a 5/2 Solenoid Valve onto a Manifold
 Polyurethane Tube Hardness Selection Errors: Three System Problems Engineers Often Overlook
Polyurethane Tube Hardness Selection Errors: Three System Problems Engineers Often Overlook
 Why do Aluminum Cylinder Barrel Need Anodizing?
Why do Aluminum Cylinder Barrel Need Anodizing?
 PVC Flexible Tubing: How to Reduce UV Aging Risks for Outdoor Use
PVC Flexible Tubing: How to Reduce UV Aging Risks for Outdoor Use
 How to Control an Angle Seat Valve with a Solenoid Valve
How to Control an Angle Seat Valve with a Solenoid Valve
You May Interest In

Jan 05, 2026 Blog
What is Plumbing and Pipe Fitting?
Jan 04, 2026 Blog
what are male and female pipe fittings?

Dec 05, 2025 Blog
FRL: A 5-Minute Illustrated Selection Guide
Nov 10, 2025 Blog
What is Pneumatic Cylinder Cushioning?
Nov 04, 2025 Blog
How to remember ester vs ether quickly?

Oct 24, 2025 Blog
8 common reasons for pressure gauge might failure

Aug 01, 2025 Blog
Complete Analysis of Pneumatic Rotary Cylinders

Jul 30, 2025 Blog
Comprehensive Guide to Compact Air Cylinders





Apr 23, 2025 Blog
Exploring the Critical Parts of a Pneumatic Cylinder
Apr 23, 2025 Blog
anti-rotation cylinder
Mar 28, 2025 Blog
Leakage Prevention and Sealing Methods in Cylinder

Mar 18, 2025 Blog
How to Install an Air Compressor Regulator
Mar 13, 2025 Blog
What is Magnetic Pneumatic Cylinders?
Mar 10, 2025 Blog
Compact Anti-Rotation Cylinder with Rod
Mar 10, 2025 Blog
Customized Long Stroke Air Cylinder for Truck
Mar 10, 2025 Blog
Customized Combination Manifold Valves


May 16, 2019 Blog
STAINLESS STEEL 316 FITTINGS, SS304, SUS316
May 03, 2018 Blog
Application Of Tube Fitting
Jun 08, 2018 Blog
Why Do You Choose Our Air Cylinder kits?
Feb 09, 2018 Blog
How much you know about globe valve?FOKCA ©1998-2026 Fescolo Pneumatic All Rights Reserved Sitemap