Do you know the terms "functional group" and "carbonyl group"? These terms are often used to describe specific atoms or groups of atoms in an organic molecule that determine its chemical reactivity and properties.
A functional group is a specific atom or group of atoms in a molecule that determines its chemical properties.
A carbonyl group is a functional group consisting of a carbon atom double-bonded to an oxygen atom (C=O), common to many compounds.
Ester vs Sther Functional Group
Esters and ethers are both common functional groups used to classify organic compounds. The main difference between esters and ethers lies in their atomic structures. An ester molecule contains two oxygen atoms and one carbon atom in its functional group, while an ether molecule contains only one oxygen atom connecting two carbon atoms.
So, how can we quickly remember the difference between ethers and esters?
Next, we will use a diagram and a table to show the structural differences between ester based and ether based in detail.
The image of the structure of Ether and Easter bond is shown in the image below.
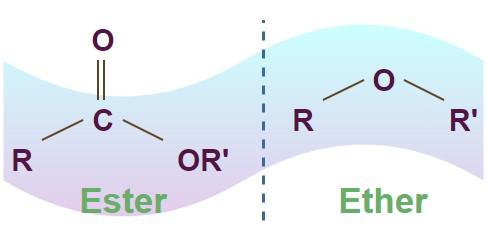
Esters and Ethers Definition
The main reason for the various physical property differences between esters and ethers lies in the difference in intermolecular polarity between the two materials. The following table mainly illustrates the differences in molecular structure and molecular properties.
Difference between Ester and Ether
|
Ester
| Ether |
| Ester do not have symmetrical structures due to the presence of the carbonyl group | Ethers can have symmetrical structures if both alkyl groups on either side of the oxygen atom in an ether group are similar. |
| Esters have the functional group -COO | Ethers have the functional group -O- |
| Ester have polar bonds | Ether has both polar or nonpolar bonds |
| A double bond between carbon and oxygen atom | A single bond between carbon and oxygen atom |
| Derived from carboxylic acids | Derived from alcohol |
| Esters that have fragrant odours are used as a constituent pf perfumes, essential oils, food flavorings, cosmetics | Ethers are relatively unreactive, and as a result they are useful as solvents for fats, oils, waxes, perfumes, resins, dyes, gums and hydrocarbons |
| Ester boiling point is 57°C | Ether boiling point is 34.6°C |
| Ester melting point is -98°C | Ether melting point is -116.3°C |
Whit ia Ester?
Esters are a class of organic compounds formed by the reaction of carboxylic acids and alcohols. Their general formula is R–COO–R′.R and R′ represent hydrocarbon groups, and the middle –COO– is the ester group.
Esters are derivatives of carboxylic acids, in which carbon atoms are bonded to single-bonded oxygen atoms. Esters are compounds formed by the combination of organic or inorganic acids. Esters are formed by the reaction of acids with alcohols. First, the alkyl or aromatic moiety provided by the "alcohol" part is named. The "acid" part is then named a carboxylic acid, and its suffix "-ic" is changed to "-ate".
Esters have a pleasant sweet aroma and are therefore widely used in synthetic fragrances, perfumes, nail polish removers, inks, pharmaceuticals, and biodiesel. Esters also have a fruity aroma.
Uses of Ester
◆ It can used as Fragrances and Flavors
◆ Esters can used as Solvents
◆ It can used as Plastics and Fibers
◆ It can used as Pharmaceuticals
◆ Ester can used as Biofuels
Whit ia Ether?
An ether group is a group consisting of one oxygen atom and two carbon atoms (alkyl groups) linked by a single bond. The general formula is R–O–R′, where R and R′ are usually hydrocarbon groups (such as alkyl or aryl). Ethers are highly volatile organic compounds.
Ethers are typically colorless, volatile liquids with low polarity and relatively stable chemical properties.
Because they do not readily undergo chemical reactions and can dissolve many organic substances, they are generally classified into two types: Symmetrical ethers (general formula R–O–R) – e.g., CH3–O–CH3 (methoxymethane) Asymmetric ethers (general formula R–O–R′) – e.g., CH3–O–CH3 (methoxyethane)
Uses of Ether
◆ It is widely used as a commercial solvent.
◆ Diethyl ether can be used as a coolant.
◆ Diethyl ether is used as an anesthetic in surgical operations.
◆ It can serve as a nonpolar solvent in Grignard reactions.
◆ Diethyl ether is used together with gasoline as a motor fuel.
Tpu ester vs ether
In the application of pneumatic tubing, the difference between ester and sther is also very obvious. Polyether polyurethane is suitable for applications that require resilience due to its flexibility, while polyester polyurethane is suitable for applications that require a certain degree of pressure resistance and strength due to its wear resistance.
Polyurethane (PU) tubing is commonly made from ester-based (polyester) or ether-based (polyether) raw materials. Both contain oxygen atoms, but their molecular structures differ, resulting in significant differences in physical properties, chemical properties, and processing characteristics, such as abrasion resistance, hydrolysis resistance, low-temperature flexibility, chemical resistance, and cost.
Visit this article to learn more difference about ester and ether.